Childhood Diabetes and a New Medicine – Part 1
I’d like to talk today about a “new med” for “juvenile diabetes.”
By ‘childhood diabetes’ I mean the ‘type 1’ diabetes that used to be most predominant in childhood as opposed to the ‘type 2’ which used to be most predominant in adulthood. Two different diseases which, confusingly, have the same name.
Diabetes – not the same disease we know today.
And, by ‘new med’ I mean a med which is ‘old enough’ to have gone completely through the US drug approval process, be put on the market, used and begin raking in the big bucks long enough for the company to want to take the risk of trying to prove its use in a new market – kids.
We’ve already discussed the fact that the bombardment of advertising, the “electronification” of social interaction and the uncertainty-protectiveness of our children have all conspired to produce a generation of alarmingly obese “couch-potato” kids.
But you may not realize that it has grown to such an extent that it required a complete change in our medical nomenclature – so much of the disease we see in children is now weight induced (the adult type) that we had to completely give up on the name “Juvenile Onset Diabetes” (JODM) and now merely call them “type 1” and “type 2.”
The ‘confusion’ doesn’t end there, however, the new drug (I’ll give you its name in a minute) acts sort of, in some ways, just the opposite of Insulin – which has been the mainstay of treatment [and a godsend, for those old enough to remember life without it] in ‘childhood’ diabetes ever since it was discovered on July 30th, 1921 by Canadian physician Frederick Banting and medical student Charles H. Best.
Treating Children With Diabetes
Juvenile Onset Diabetes Mellitis
Forgive me, but I don’t hold any more hope that you’ve got a better grasp on how substantial a disease “Juvenile Onset Diabetes Mellitus” (JODM – Type 1) is than you have on diseases like Congenital Rubella, Measles, Polio or Small Pox – which isn’t very great.
But, in your defense, they just aren’t the same diseases they once were.
There once was ONLY ONE diabetes, Juvenile Onset, and it had ONLY ONE outcome – death… usually within months of onset. Besides all the “patented” quack “cures” scammed on the hapless and desperate by the original “what-your-doctor-doesn’t-want-you-to-know” con-artists of the day, there was really ONLY ONE known “treatment,” a draconian diet – and NO known cure.
About all the doctors knew at the time was that sugar and sweet foods made the condition worse; so, putting patients on a very strict diet which kept sugar intake at a minimum was their only hope, at best gaining them only a year or so extra life. In some cases, the harsh diets even caused patients to die of starvation.
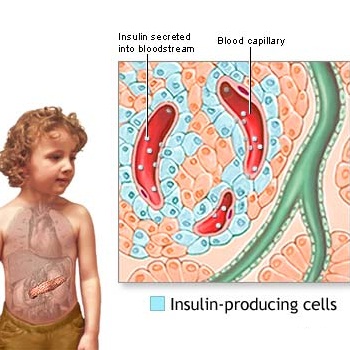 Pancreas and Islets of LangerhansExcruciatingly slow observation and experience revealed first that those who died frequently had damaged pancreases. That organ already was known to help digestion but in 1869 a German medical student, Paul Langerhans, discovered a second function.
Pancreas and Islets of LangerhansExcruciatingly slow observation and experience revealed first that those who died frequently had damaged pancreases. That organ already was known to help digestion but in 1869 a German medical student, Paul Langerhans, discovered a second function.
Within the mainly digestive organ there were tiny islands of totally different cells, “islets” if you will, which eventually were shown to produce the “insulin” needed to process sugar.
When the true function of these cells became known, they were named after their discoverer: the “islets of Langerhans.” Twenty years later, still in Germany, Oskar Minkowski and Dr. Joseph von Mering proved it was damage to the islet cells which caused diabetes.
Slow Discovery of Insulin
Still forty years later in 1921, Frederick Banting, an obscure Canadian “surgeon” (with a bachelor’s degree in medicine) went to the then leading authority, professor J.J.R. Macleod, with a proposal on how to obtain the “unknown sugar control substance” from the pancreas of dogs (the most common research animal back then – next to rats.)
Macleod poo-pooed Banting’s theories; but, was pressed upon to yield some laboratory space, ten experimental dogs and a medical student assistant, Charles Best.
Banting and Best first removed the pancreas and found that the dog’s blood sugar rose; it became thirsty, drank lots and urinated lots; and it became weaker and weaker – the exact symptoms of diabetes.
Then they prepared another pancreas, half-froze it, ground it up and filtered it to extract a substance they named: Isletin. When injected into the diabetic dog (by then near death) it revived and thrived! They had found a treatment! Now, if they could just find a more ready source of the stuff.
 Banting, Macleod, Best and CollipMacleod then decided that he could afford to be a little impressed with their ideas, but demanded extended experimentation before he would help them publish their work. He gave them a bigger lab and wanted them to call the stuff: Insulin. Eventually they switched to using pancreases from slaughtered cattle which seemed to work just as well.
Banting, Macleod, Best and CollipMacleod then decided that he could afford to be a little impressed with their ideas, but demanded extended experimentation before he would help them publish their work. He gave them a bigger lab and wanted them to call the stuff: Insulin. Eventually they switched to using pancreases from slaughtered cattle which seemed to work just as well.
Insulin Treatment
They brought on a biochemist, Bertram Collip, whose job it was to purify the insulin extract enough that it could be used in humans, and he did. Now they needed to try it on humans, and who better than themselves.
Doing so they got dizzy with symptoms of LOW blood sugar and learned how to control overdose symptoms with orange juice and honey as well as a more correct dosage. Now (1922) they were ready to try it on a 14 year-old boy with JODM, Leonard Thompson, who lay nearby close to death from the disease.
They boy revived, other volunteer diabetics did just as well and the Eli Lilly company perfected production enough – at a cost reasonable enough – that thousands upon thousands of people could now largely escape the terrible ravages of the disease; but alas, there was still no cure.
Glucagon
The engineers among you might be a little perplexed by this simple explanation and wondering how a single feedback-loop between blood glucose, the pancreatic islet cells and insulin secretion could possibly be sensitive and rapid enough to keep blood sugars to the close tolerance needed to maintain health.
Well, we now know it’s not as simple as we once thought and does have several alternate pathways that play into the blood sugar level equation and provide plenty of “checks and balances” to the system – as well as other places that things can go wrong.
One of these is the peptide Glucagon, a hormone which is also produced in the pancreas, only by the “alpha cells,” instead of the “beta cels” where insulin is made. And it has the opposite effect of insulin on blood sugar – it raises it should it get too low.
They do NOT cancel each other out as one might expect, but have a synergistic effect on the body. Insulin lowers the sugar in the blood by helping it get out of the blood and into most body cells. Glucagon raises the blood sugar by helping the body extract sugar from storage and to make new sugar. So, the Pancreas releases insulin when the blood sugar is too high and Glucagon when it gets too low.
Both compounds have been synthesized, so individuals with diabetes have both insulin and glucagon in their arsenal of treatments should they need it. Being a bit cumbersome to store and mix, glucagon – which breaks apart glycogen stored in the liver into its parts: straight glucose – is most often used only after more easy sources of glucose like orange juice and honey have been tried.
Glucagon-like Protein-1
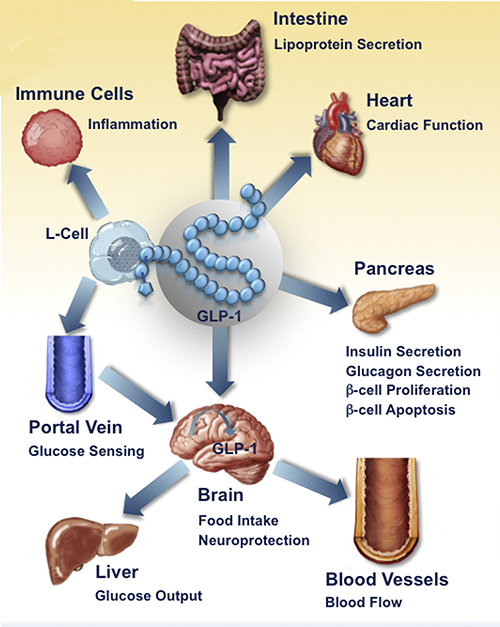 Effects of Glucagon-like Protein 1And that leads us to a whole other class of “sugar regulators” which have been capturing the hearts of diabetes researchers for the past few years: Glucagon “like” proteins, made down in the lining of the gut.
Effects of Glucagon-like Protein 1And that leads us to a whole other class of “sugar regulators” which have been capturing the hearts of diabetes researchers for the past few years: Glucagon “like” proteins, made down in the lining of the gut.
Finding these little devils has been a real chore for many years. You see, we’ve long seen a puzzling effect that raising a persons blood sugar by placing glucose directly into the vein doesn’t cause near as great an increase in insulin production as does placing that same amount of glucose into the gut and letting it be absorbed that way. Something in the gut was causing an enhanced effect!
Then, when gastric bypass surgeries began to be performed, we noticed that some patients whose metabolism was greatly out of whack slowly showed unexpected improvement after the surgery. Again, something was in the gut messing with blood sugar levels over and above insulin and glucagon.
It turns out that those little intestinal L cell’s have been secreting “Glucagon-like Protein 1” (GLP-1) as a gut hormone and it has a whole host of effects:
- It increases insulin secretion from the pancreas in a glucose-dependent manner.
- It decreases glucagon secretion from the pancreas.
- It increases insulin-sensitivity in both alpha cells and beta cells.
- It increases beta cells mass and several insulin enhancements.
- It inhibits acid secretion and gastric emptying in the stomach.
- It decreases food intake by increasing satiety in brain.
- It promotes insulin sensitivity.
Where has this stuff been all our lives, and how do we get some more!
And, finally, that now brings us precisely to the recent development that I’ve been trying to get around to telling you about all this time.
One of those new-fangled drugs which act like “Glucagon-like Protein 1” (GLP-1), and which already has been released for use in Type 2 (adult) diabetes some time ago, has now been shown to be useful in Type 1 Diabetes (juvenile) as well – like many of us thought it would be.
However, I’ve yammered on so that I’ve run out of space and will now need to take this discussion up again on “page two.”
[American Association of Clinical Endocrinologists 23rd Annual Scientific and Clinical Congress. Presented May 16, 2014]
2 Posts in Childhood Diabetes (diabetesmed) Series
- Part 2 - GLP-1 Meds – 27 Jun 2014
- Part 1 - Diabetes history, GLP-1 – 23 Jun 2014

