Topical Gel Helpful in Some Infantile Hemangiomas
We all know that birthmarks aren’t very uncommon. One particular type, Infantile Hemangiomas, can kind of sneak up on parents and some times be quite worrying.
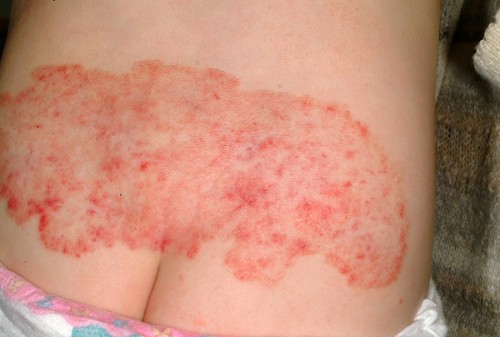
About four percent of infants develop these kinds of lesions and most of them don’t become visible until sometime after birth. They usually increase in size until around nine months of age; then, eventually, decrease in size between 2 and 10 years. Once in awhile they don’t regress fully.
They can, but usually don’t, have a physical effect on a child. More often they have an emotional effect on the child and family; but, the two don’t necessarily go together. How they are seen is more determined by how they are “seen” – visibility: their location and size.
There are drugs that are used to treat more severe hemangiomas and when they work it can be quite rapidly – a matter of days. However, the drugs are not without risk of side effects and their use in small or minor lesions just isn’t worth the risk – even if they don’t look good and are bothersome.
Some researchers in Australia have recently reported what they found when they studied children with lesions using a similarly acting drug but in a gel form which can be applied to the skin, thus having less side effects.
Located at the Sydney Children’s Hospital in Randwick, Australia, Dr’s Hsien Chan and Orli Wargon closely followed 41 infants, between 5 and 41 weeks of age, who had superficial focal hemangiomas without ulceration and not near mucosal surfaces.
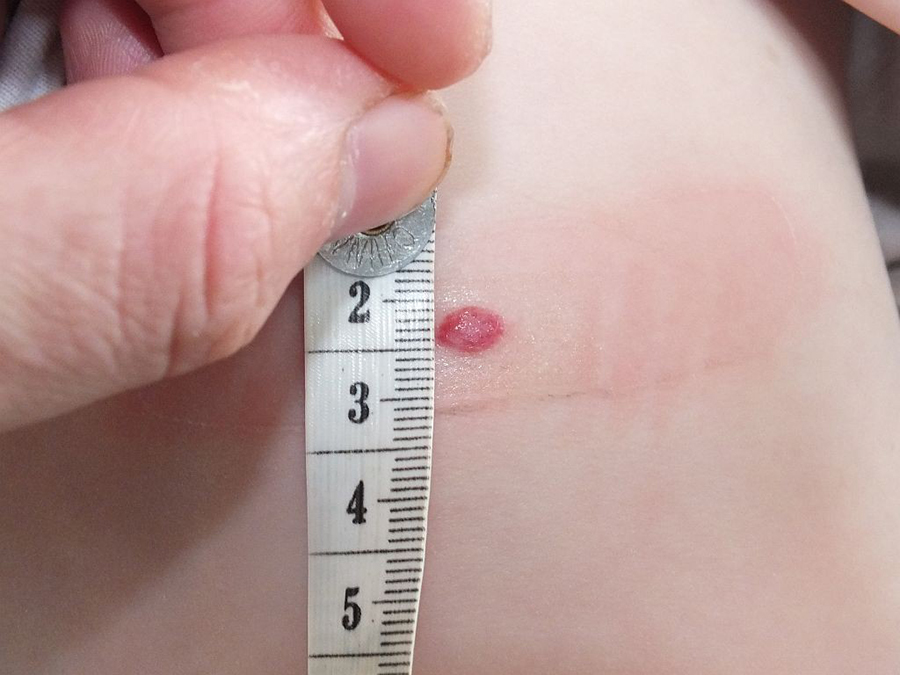 The children were evenly and randomly treated with either placebo or Timolol gel and the researchers watched for either a response or the occurrence of side-effects. While oral drugs used in larger lesions cause a response fairly rapidly, effects weren’t seen in this study until after 12 to 16 weeks.
The children were evenly and randomly treated with either placebo or Timolol gel and the researchers watched for either a response or the occurrence of side-effects. While oral drugs used in larger lesions cause a response fairly rapidly, effects weren’t seen in this study until after 12 to 16 weeks.
We need to keep in mind that a study like this is very complicated to do because these children are of an age where their lesions are “supposed” to be changing in size anyway – whether they get any drug or not. Therefore, the doctors needed to do some calculating of “expected size” over time and correct their findings for that.
Of the 41 children, 37 completed the study to its 24-week end. Nine children withdrew from the study, and there was evidence that impatience with gel treatment was a factor in these withdrawals. Seven of those nine subsequently received the “quicker but riskier” oral propranolol.
What the doctors found, and reported about in the May edition of Pediatrics, the medical journal, was that after week 16 there was a significantly lower predicted percentage volume increase in the treatment group than in the placebo group.
To make sure they were evaluating the lesions correctly they also took detailed photos and sent them to unrelated physicians for their “blinded” evaluation of progress. At 24 weeks, 47% of the gel treated children were free of redness compared with only 6% of placebo treated infants. In addition, only 6% of the actively treated group were deemed “completely red” whereas 55% of placebo patients were judged that way.
So, there was not only a significant reduction in volume (confidence level: P = .02 – good) but a loss of redness as well (confidence level: P = .01 – even better); and all without seeing any episodes of bradycardia (slow heart rate) or hypotension – the two worrisome side-effects that I spoke of earlier.
This topical gel treatment was deemed to work the very best on lesions whose diameter was under 11.3 millimeters (one half inch) but, according to their findings “is a safe and effective way of treating superficial infantile hemangiomas not meeting the criteria for systemic treatment.”
Pretty good news!
[Pediatrics. Published online May 6, 2013.]
Advertisement by Google
(sorry, only few pages have ads)